Introduction
Thermodynamics is a field of science that deals with the relations between heat, temperature, and work, as well as their interaction with energy, radiation, and the physical properties of matter. The movement of atoms and molecules within an object generates heat. The faster the molecules move, the more heat is produced. The energy thus produced in the form of heat is called thermal energy. However, thermodynamics is not concerned in particular, with the rate of energy transformations. In fact, it is concerned with the macroscopic properties of matter. It is concerned with the entire bulk system, rather than the molecular makeup of the system involved.
Just like all other branches of science, thermodynamics has its own set of terms, concepts, and sign conventions that help us navigate through it. A thorough comprehension of all these concepts ensures a healthy foundation for one’s understanding of the subject. It also helps to avoid misunderstandings regarding the subject later down the line. For instance, it becomes imperative to define a system and its surroundings because it is the basis for many fundamental descriptions and calculations in thermodynamics. Such concepts of thermodynamics will be covered in this article.
Basic Concepts Of Thermodynamics

basic-concept
System+Surrounding => Universe
1. System
A system of thermodynamics can be defined as any matter or any specific region of space that is chosen for analysis. The system is the “matter of investigation” in thermodynamics. A system can be distinguished from its surroundings by a boundary. Such a boundary can be real or imaginary, and fixed or free to move.
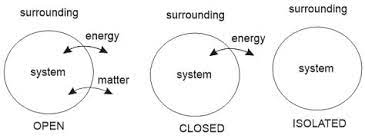
In physics, a material or a system is said to be “homogenous” when it is composed of the same properties throughout. However, a system is “heterogeneous” when parts of it vary in physical or chemical properties. Based on how energy and/or mass can travel in and out of a system, it can be divided into three types:-
Open System:
Closed System
Isolated System
![types-of-system]()
types-of-system
Types of System
Open System– An open system is one that can exchange matter and energy (work/heat) between itself and its surroundings. Open systems are sometimes known as “flow systems” because of their ability to exchange the mass of a system. All biological systems are open systems as they consume energy-storing molecules and release energy to the environment by work done.
![open-system]()
open-system
Examples of an Open System:
The human body
Automobile engines, steam turbines
An open flask containing hot coffee
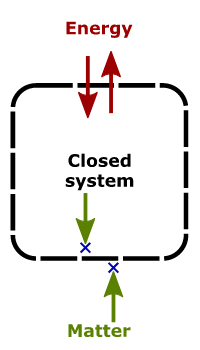
Closed System– A closed system is capable of energy exchange but cannot exchange matter with its surroundings. The transmission of energy happens across the boundary of the system. Whereas, mass is conserved. Such a system is sometimes called “non-flow processes” for its inability to exchange mass.
![closed-system]()
closed-system
Examples of a closed system:
Refrigerators
Hot coffee in a closed steel flask– only heat can transfer across it, the vapours remain conserved due to covering
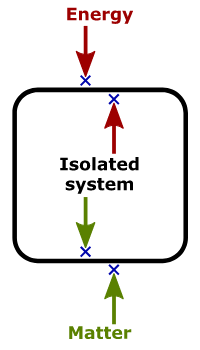
Isolated System– An isolated system can neither exchange energy nor mass with its surroundings. Such a system can be viewed as one that is completely sealed to avoid spillage of matter, and thermally insulated to prevent heat flow.
isolated-system
Examples of an isolated system:
The physical universe is said to be isolated
Hot coffee in a sealed thermos flask
2. Surrounding
The remaining portion of the universe, that is not included in the concerned system, is known as the surrounding. Surrounding is also defined as everything outside the system boundary that has a direct impact on the behaviour of the system. For instance, the air around a piston-cylinder assembly is its surroundings.
Together, a system and its surroundings make up the universe.
Heat (Q), Work (W), And Internal Energy (U)
Heat is the form of energy transferred from one system to another, where both can be distinguished by a difference in their temperature. Heat, as a form of energy, can neither be created nor be destroyed. It can only be transferred from one location to another. Heat energy, also referred to as thermal energy, can be transferred from a system at a higher temperature to a system at a lower temperature. Heat energy also corresponds to the quantifiable mechanical work done on or by a system.
Heat transfer (Q) and work done (W) are the two common means of gaining or losing energy, in or out of a system. The two processes, however, are different from each other. Heat transfer is a less organised process driven by temperature differences. Work, on the hand, is quite an organised process driven by force exerted.
Nevertheless, heat and work can both yield identical results in practice. For example, both can result in a rise in temperature. Once such a temperature rise has occurred, it’s impossible to identify the cause as the heat transferred or the work done on it. Like a cyclist pumping air (doing work) into the tires, versus, the sun increasing the temperature (heat transfer) of air in the tires. This uncertainty is important to note because both of these are capable of changing the internal energy (U) of a system.
Internal energy can be defined in two different but consistent ways. First, the internal energy is the sum of kinetic and potential energies of all particles in a system. Thus internal energy is the sum of atomic and molecular mechanical energies.
Second, macroscopically, the internal energy of a system can be defined by the first law of thermodynamics. According to the first law, the change in internal energy is equal to the net heat transfer into the system minus the net work done by the system.

surrounding
The First Law of Thermodynamics
Equation of the first law of thermodynamics: ΔU= Q - W
Where,
ΔU= the change in internal energy U of the system
Q= the net heat transfer into the system / the sum of all heat transfer into and out of the system
W= the net work done / sum of all the work done on the system or by the system
If you desire to understand the conversion of thermal energy into useful work done, then the principle of conservation of energy is important. The first law of thermodynamics applies the principle of conservation of energy to systems where heat and work done are the methods of transferring energy in and out of the system.
Sign Conventions Used For Heat and Work In Thermodynamics
In order to avoid errors in experimental and scientific work, sign conventions are devised in thermodynamics. A sign convention is an agreeable choice of signs/symbols, such as plus or minus, for a set of parameters. In thermodynamics, we use the following sign conventions:
Conclusion
Thermodynamics is a particularly essential branch of physics and chemistry. It deals with the study of energy, its conversion, as well as the various forms and abilities of this energy to get work done. Thermodynamic concepts help you in understanding how heating and cooling systems in our homes work. It teaches us how to be energy-efficient while using these devices in daily life. It also assists in the investigation of automobile engines.
The rules of thermodynamics provide you a deeper insight into the process by which energy is turned into heat, which in turn is produced in the form of useful work.
Various thermal systems can be analysed thermodynamically, through the application of some governing conservation equations. Namely, Conservation of Mass, Conservation of Energy ( first law of thermodynamics), the second law of thermodynamics, and property relations. The energy of a system can be viewed as its ability to cause changes.